Guidelines for preparing submissions to the Pharmaceutical Benefits Advisory Committee (PBAC)
Version 5.0
September 2016
The PBAC Guidelines explain in detail how to prepare a submission to list a new medicine or medicinal product on the Pharmaceutical Benefits Schedule (ie for public funding). The guidelines provide detailed instructions on what information is required by the PBAC and the Economic Sub-Committee (ESC) to support a proposed new medicine, and the most appropriate form of clinical evidence and economic evaluation for specific submissions.
Whilst submissions to the PBAC will now need to follow the guidance provided in the PBAC Guidelines version 5.0, the PBAC has approved a transitional arrangement of two PBAC cycles in regard to the structure of submissions. That is, submissions to the PBAC for consideration at either the March or July 2017 PBAC meetings may follow the structure of either version 4.5 or version 5.0 of the Guidelines. Submissions for consideration at the November 2017 meeting must follow the structure of version 5.0 of the PBAC Guidelines.
For information on processes, procedures, timelines and documents required, refer to the Procedure guidance for listing medicines on the Pharmaceutical Benefits Scheme.
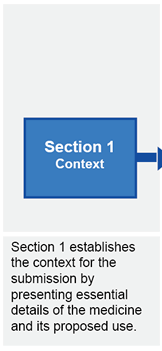
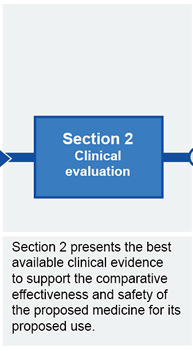
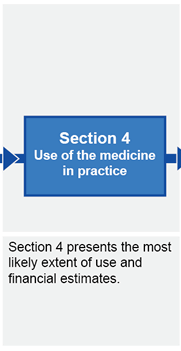
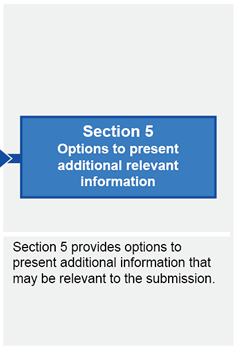
Submission structure for submissions requiring evaluation
PBAC Help centre for making a submission
Contact
Enquiries about PBAC submissions can be mailed or emailed to the Pharmaceutical Evaluation Branch at the following address:
Pharmaceutical Evaluation Branch
MDP 910
Department of Health and Aged Care
GPO Box 9848
Canberra ACT 2601
Email: PBAC@health.gov.au